I. Introduction

II. How is gold analyzed?
- On gold grade (define ore reserves, optimize mill feed).
- On the different status of gold (free, attached, included or refractory) to define plant performance and loss in tailings).
- On its speciation (under what mineralogical form gold occurs, native gold, or alloy).

Thus, the choice of the most appropriate method to determine the nature and gold content of a sample depends on its physical, chemical and especially mineralogical properties as well as the purpose of the analytical results (mining research, optimization of the treatment process, sale of concentrate, etc.).
Before the decisive step of the analysis, it is important to underline that gold ores are characterized by a great variability and that a great vigilance should be taken during the preparation of the samples. This being said, the three most commonly used methods are :
1. Fire Assay
Fire assay is a standardized technique for concentrating precious metals. The sample is first mixed with a fluxing agent, then heated to high temperature (melting) with collection by lead. A mixture of precious metals is obtained which are then extracted by a process called coupellation. The metals are then solubilized for determination by atomic absorption spectrometry or inductively coupled plasma spectrometry.
It is important to note that fire assay is frequently used as the first choice when analyzing large quantities of gold. However, the presence of sulfides, chromium metal oxides and other elements can decrease the recovery rate of precious metals and interact with the fire assay process. Indeed, to improve recovery, mining laboratories adapt the nature of the flux according to the mineralogy of the samples.
2. Acid digestion with aqua regia followed by analysis by Atomic Absorption Spectroscopy
The determination of gold by chemical digestion can be a reliable alternative to fire assay. This digestion is performed by aqua regia (aqua regia), which is a mixture of concentrated hydrochloric acid (HCI) and nitric acid (HNO3). The results of this analytical method for gold correlate fairly well with those obtained by fire assay, and if the samples have been properly pretreated. Indeed, samples that contain carbon in the form of graphite or charcoal must be roasted before digestion because gold can adsorb (preg-robbing) when dissolved, which affects the analytical results.
3. Cyanidation
Cyanidation is an indirect method of assaying gold. It consists of dissolving gold when it is only in the form of free or attached grains, thus accessible to a dilute cyanide solution. This solution loaded with dissolved gold is then analyzed to determine its concentration.
4. Mineralogical analysis
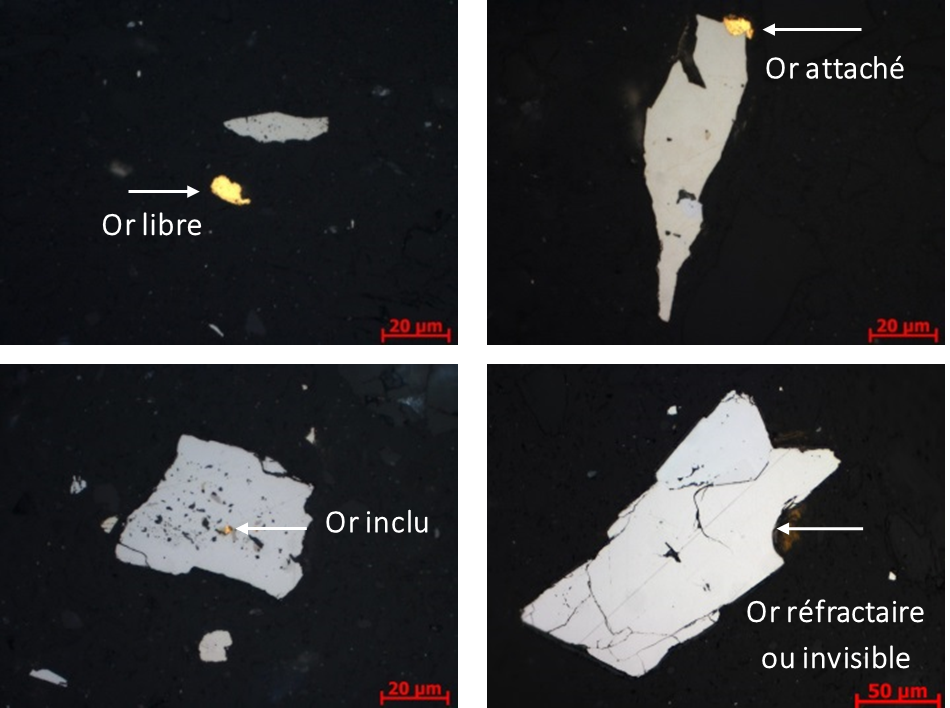
Mineralogical analysis of gold ore by microscopic observations (optical and electron microscope) and by elemental microanalysis (electron and ion microprobe, Laser ablation coupled with ICP-MS) is paramount to understand in what form is present the gold in the ore, unlike the previous analyses which only give its total content. Microscopic observations allow us to know the different statuses of gold as shown in the imagery below :
- Free gold is that which can be recovered gravimetrically
- Free and attached gold is that which can be recovered by cyanidation.
- Included gold cannot be recovered by cyanidation, but can be recovered gravimetrically if its size is significantly larger than that of the host mineral
- Refractory (or invisible) gold is gold that is embedded in the crystal lattice, and is not observable by microscopy. It is the elemental microanalyses that allow it to be detected and measured.
.